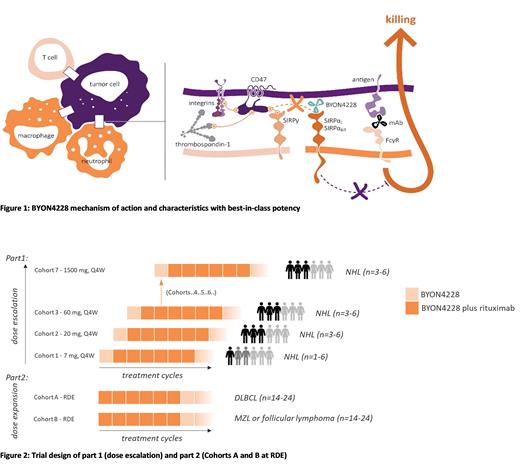
Introduction: Blocking of the immune checkpoint CD47-SIRPα promotes myeloid cell-mediated innate as well as adaptive immunity towards tumor cells. Antibodies in development are either directed against CD47 or SIRPα and in combination with tumor-targeting antibodies, checkpoint inhibitors, chemotherapeutics or other agents. The humanized SIRPα antibody BYON4228 (van Helden et al (2023) JITC 11: e006567) has been designed with three distinguishing characteristics compared to other CD47- or SIRPα-directed therapies which makes BYON4228 potentially best-in-class:
1.High specificity for SIRPα and no binding to SIRPγ on T cells which enables maximal contribution of T cells and therefore maximal anti-tumor immune response;
2.Silenced Fc backbone (IgG1-L234A/L235) which potentially results in reduced toxicity as confirmed in nonclinical studies showing no anemia or neutropenia in monkey;
3.Binding to both allelic forms of SIRPα (α 1 and α BIT) covering a broad patient population.
Phase 1 clinical trial design: BYON4228.001 (NCT05737628) is the first-in-human clinical trial of BYON4228, in combination with rituximab, in relapsed or refractory (R/R) B-cell Non-Hodgkin's lymphoma (NHL). Part 1 (dose escalation) evaluates the safety, tolerability and Pharmacokinetics (PK) of, and receptor occupancy by BYON4228 to determine the maximum tolerated dose (MTD) or optimal biological dose (OBD) if the MTD is not reached, and recommended dose for expansion (RDE). A 28-day flat dose escalation schedule starting at 7 mg will be followed in a standard 3+3 design. Notably, only 1 cycle BYON4228 monotherapy will be given in each patient before combining with rituximab (375 mg/m 2 first cycle weekly administration; thereafter every 4 weeks for a total of 6 cycles). In part 2 (cohort expansion) BYON4228 + rituximab combination therapy will be given from cycle 1 onwards to further evaluate safety and efficacy in up to 24 additional diffuse large B cell lymphoma (DLBCL) patients and up to 24 patients with either marginal zone lymphoma (MZL) or follicular lymphoma (FL).
This trial design of only 1 cycle monotherapy and swiftly moving into combination therapy not only allows to investigate PK and safety of BYON4228 but also efficacy of combination treatment with rituximab in a relevant therapeutic setting.
Enrollment is ongoing in The Netherlands, United Kingdom, Italy and Spain since June 2023.
Key inclusion criteria include, for part 1: B-cell NHL expressing CD20 by immunohistochemistry (IHC) or flow cytometry, R/R to at least 2 prior lines of therapy; for part 2, cohort A: Histologically confirmed DLBCL expressing CD20 R/R to front-line therapy, or second-line salvage regimens; for part 2, cohort B: Histologically confirmed MZL or FL (Grade 1-3a) expressing CD20, R/R to at least 2 prior lines of therapy. Autologous or allogeneic hematopoietic cell transplantation and autologous CAR-T cell therapy are allowed as prior lines. Key exclusion criteria include prior treatment with CD47- or SIRPα- targeting agents at any time; other anticancer therapy including investigational agents within 2 weeks or within at least 4 times the elimination half-life; Burkitt's lymphoma; history of active auto-immune disorders, active infections or uncontrolled systemic disease. Tumor response is determined according to Lugano and LYRIC criteria. An extensive biomarker program in the peripheral blood and bone marrow is included in the trial.
Disclosures
Klaassen:Byondis: Current Employment. van den Berg:Byondis: Current Employment. van den Tweel:Byondis: Current Employment. Hooren:Byondis: Current Employment. Schellens:Byondis: Current Employment.